Check out our advocacy in the community and beyond, our efforts help to elevate food allergy as a condition with the government, industry, and healthcare providers. Learn how you can participate in a research study for children with peanut allergy, and discover the latest research updates on food allergy treatments and therapies, and epinephrine nasal sprays.
Advocacy: Highlighting the need for the National Food Allergy Action Plan with the federal government

Members of the House of Commons Standing Committee on Finance were in Toronto last month to hear from individuals and organizations about their budgetary priorities. Jennifer Gerdts, our Executive Director addressed the Committee on the need for federal support for the over 3 million Canadians impacted by food allergy, aligned with our 2024 pre-budget submission focused on our National Food Allergy Action Plan.
This opportunity allowed us to connect directly with key stakeholders in government and call on them to help support the priority issues faced by our community.
We want to thank the Committee Chair, Peter Fonseca and MPs Gabriel Ste-Marie, Yvan Baker, Julie Dzerowicz, Adam Chambers, Philip Lawrence, and Matthew Green for considering our request in support of our community!
Advocacy: Managing food allergens is a food safety priority
This fall, we participated in the annual Food Safety Symposium, hosted by the Ontario Food Protection Association.

This annual event brings together members of the food industry in Ontario, focused on food safety issues, including food allergens. Our Director of Food Safety & Regulatory Affairs, Beatrice Povolo, conducted a session on the importance of effective allergen management practices and labelling for food manufacturers, and the effect this has on the community impacted by food allergy.
Helping to inform the food industry on issues that directly affect our members is a focus of our food safety agenda and events like this help us reach our key stakeholders in food manufacturing and foodservice.
We continue to be your voice at the table to make sure your needs are heard and ensure you have access to accurate ingredient information to make informed decisions.
Advocacy: Elevating food allergy as a health condition with family doctors and others

This year, we participated in key healthcare conferences, like the Family Medicine Forum which had over 3,500 family doctors coming together in November. At this conference, Jennifer Gerdts, our Executive Director, and Dr. Ben-Shoshan presented a hot topic session on “Navigating Non-IgE-Mediated Food Allergy”. At each conference we attend, we ensure the physicians receive our materials so they not only become informed but can share these resources with their patients.
Our efforts at healthcare conferences help to elevate food allergy as a medical condition so all Canadians impacted by food allergy are better supported. This is particularly important in rural and remote regions where access to allergists may be difficult.
Research study – Call for participants: Children with peanut allergy
A research study is underway across North America. They are seeking children aged 4-7 years who have been diagnosed with peanut allergy and are currently following a strict peanut-free diet.

The study is testing an investigational drug patch to learn how well it works and how safe it is in children with peanut allergy. The purpose of the patch is to potentially desensitize a person with peanut allergy by repeated exposures to very small amounts of peanut via the skin.
Following is an announcement from the research team. You can also visit VitesseAllergyStudy.com for more details and view their FAQ sheet. Please note, study sites will continue to be added, so check back often.
Please share with others whose children have peanut allergy.
From the research team:
Now Enrolling: The VITESSE Phase 3 Study for Peanut Allergy
The VITESSE phase 3 clinical research study is looking for children 4 to 7 years of age who have been diagnosed with peanut allergy and are currently following a strict peanut-free diet. Study doctors are testing an investigational drug patch (also called study drug patch) to learn how well it works and how safe it is in children with peanut allergy.
What should I know about the VITESSE study?
- To be eligible for this study, participants must be*:
- 4 to 7 years of age
- Diagnosed with peanut allergy
- Currently following a strict peanut-free diet
*Other inclusion/exclusion criteria will apply.
- This study will consist of at least 12 study visits and 5 phone calls over a period of approximately 58 weeks (about 1 year)
- Participants will be randomly assigned (by chance) to receive the study drug patch or placebo patch (looks like the study drug but contains no active drug). Participants will have about a 67% (2 in 3) chance of receiving the study drug patch and about a 33% (1 in 3) chance of receiving the placebo patch
- The health and safety of participants will be monitored throughout the study
- Participant data and information will be kept confidential according to applicable laws for clinical research studies
- Study participants will receive all study-related procedures and the study drug patch or placebo patch at no cost
To learn more about the VITESSE phase 3 study and eligibility criteria, please visit VitesseAllergyStudy.com or ClinicalTrials.gov (identifier: NCT05741476).
Study sponsored by DBV Technologies.
Research: The latest research updates on food allergy treatments and therapies
There are different forms of food allergen immunotherapy being studied in clinical trials. These trials are often done in four phases and each phase is designed to answer certain questions. We have listed a few of the latest trials for you below.
These developments in food allergy treatments and therapies are encouraging, and we’ll continue to share the latest information with you.
Viaskin™ Peanut for treatment of peanut allergy

DBV Technologies has announced positive 2-year results from its Phase 3 trial evaluating Viaskin Peanut in toddlers. Viaskin Peanut is based on epicutaneous immunotherapy (EPIT) involving a patch containing peanut protein that is applied to the skin. According to study results, an additional 12 months of treatment (24 months total) with the VP250 skin patch, containing the equivalent of approximately 1/1,000 of a single peanut, increased the percentage of toddlers able to tolerate peanut.
Read their press release for more information.
Multi-allergen therapy receives U.S. FDA Fast Track Designation
Alladapt Immunotherapeutics has announced that ADP101, a multi-allergen oral immunotherapy (OIT) designed to treat allergy to one or more food allergens, has received Fast Track Designation from the U.S. Food and Drug Administration (FDA).

Previously, the company announced encouraging Phase 1/2 results in pediatric patients allergic to one or more food sources in the product; the Fast Track designation for ADP101 was supported by these results.
Fast Track is an FDA process designed to expedite the development of new drugs intended to treat serious or life-threatening conditions.
Read their press release for details on the FDA Fast Track Designation and their press release for more information on the Phase 1 /2 results.
Toothpaste immunotherapy for treatment of peanut allergy

In November 2022, we shared news about a specially formulated toothpaste containing peanut protein, INT301, under development by Intrommune Therapeutics.
The Phase 1 clinical trial involving adult patients has been completed, and last month, results were released demonstrating the toothpaste can safely be used to deliver oral mucosal immunotherapy (OMIT). The company plans to report on the full data set next year, followed by initiation of the Phase 2 trial involving pediatric patients.
Read their press release for more information.
Research: Updates on epinephrine nasal sprays
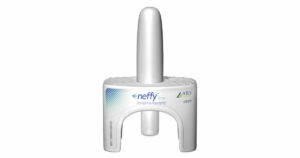
ARS Pharmaceuticals presented clinical data supporting its neffy® epinephrine nasal spray as a safe and effective option across patient sub-populations. The company anticipates a resubmission to the U.S. Food and Drug Administration (FDA) in the first half of 2024, positioning its nasal spray for an FDA action date in the second half of 2024. Read their November press release and September press release for more information.
Bryn Pharma announced positive data supporting the use of its epinephrine nasal spray in adults with or without congestion. Nasal congestion as a symptom of anaphylaxis was studied as it could affect the absorption of a nasal spray. The company is working to continue the advancement of a development program focused on efficacy and safety comparisons of its spray to the current standard of care treatment, the 0.3 mg epinephrine auto-injector. Read their press release for more information.

To learn more about needle-free epinephrine for treating anaphylaxis, check out our Listen & Learn episode with Dr. Harold Kim.
As the news on needle-free epinephrine products for treatment of anaphylaxis continues, we’ll keep you updated.
