Identifying priority allergens in natural health products such as vitamins, minerals, probiotics, herbal remedies, homeopathic and traditional medicines will now be made easier and help you make safer choices!
Advocacy in action

We are pleased to let you know that Health Canada recently announced new amendments to the Natural Health Products Regulations, which now include enhanced labelling for priority food allergens.
Enabling access to accurate ingredient information is essential to managing food allergy and is a core focus of our organization. Over the past 5 years, we have engaged in ongoing consultations with the Natural and Non-prescription Health Products Directorate to ensure the labelling of priority food allergens is required on natural health product labels.
Following the public consultation in the summer of 2021 on the proposed regulatory amendments, Health Canada has now published the Regulations Amending the Natural Health Products Regulations in Canada Gazette II and an accompanying Guidance document: Labelling of natural health products for industry. We’d like to thank everyone who responded to our invitation and participated in the consultation last summer. Your collective voices paved the pathway for change.
Here are the key highlights of the new regulation amendments:
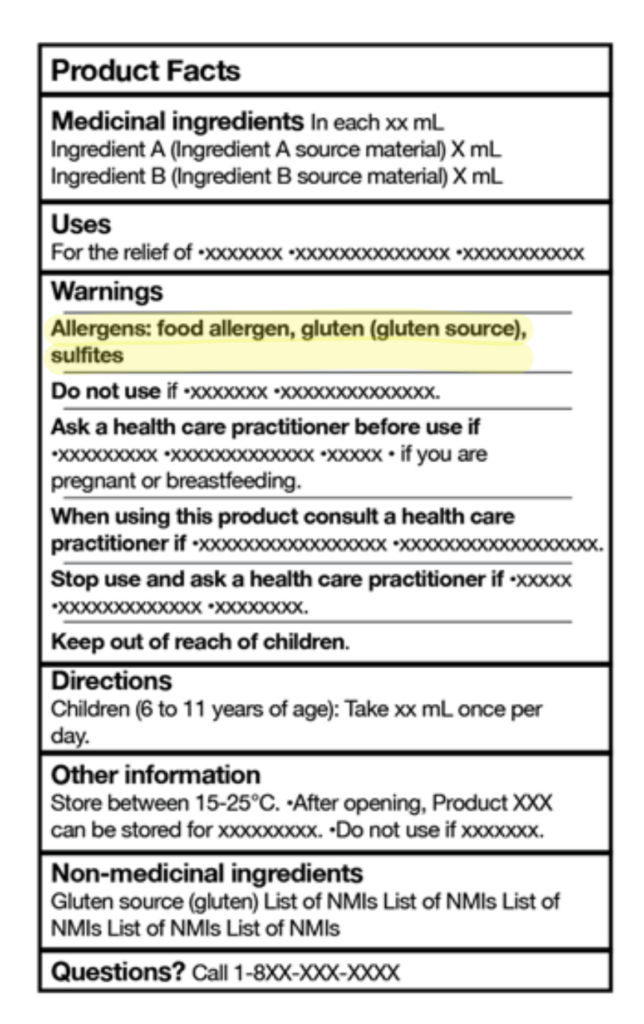
New allergen alert section has been added to the label – All priority allergens must now be listed in the new “Allergens” section on the product label. For example, if the product contains milk, wheat, and sulphites, the statement must appear in bold as follows:
Allergens: milk, wheat, sulphites
Common names of ingredients are required – priority allergens used as eithermedicinal or non-medicinal ingredients must be listed in common language
(e.g., casein (milk))
Ingredient information must be available regardless of whether you purchase online or in store – this will help ensure you have the ingredient information you need to make an informed choice at point of purchase
Coming into force
With any regulatory change of this scope, manufacturers are given time for implementation so you will begin to see these changes over the next few years starting in 2025, with full compliance by 2028.
As always, we continue to advise consumers to read the label on any products being purchased including reading both the medicinal and non-medicinal ingredient statements on natural health products and if you have any questions, contact the manufacturer directly.
Tags: advocacy, Advocacy in action, food allergen labelling, labelling, natural health products
